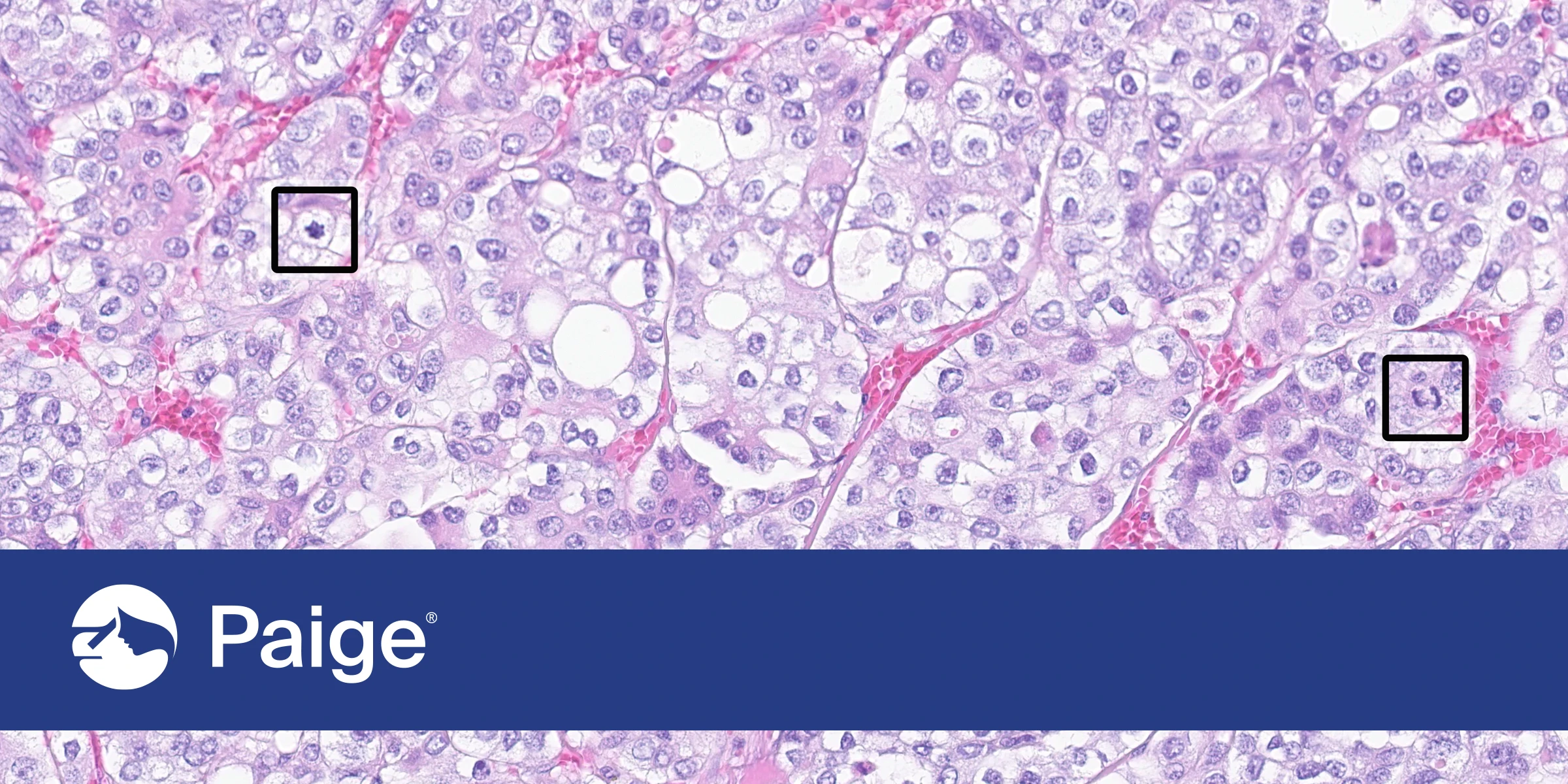
Paige’s Robust Suite of AI-Powered Applications Delivers Greater Accuracy, Confidence and Efficiency for Breast Cancer Diagnosis and Reduces the Burden of Manual Diagnosis
NEW YORK–(BUSINESS WIRE)–Paige, a global leader in end-to-end digital pathology solutions and clinical AI applications that assist in diagnosing cancer, today launched the enhanced Paige Breast Suite, a complete set of products designed to empower pathologists in their diagnosis of breast cancer, reduce the subjectivity and tedium of manual diagnosis, and increase diagnostic efficiency and confidence. The Paige Breast Suite includes Paige Breast Detect, Paige Breast Neoplasm, Paige Breast Mitosis, Paige Breast Lymph Node and HER2Complete, a comprehensive group of AI tools that help to support every step of breast cancer diagnosis and to streamline pathologists’ day-to-day workflows.
The Suite offers a state-of-the-art AI-powered tool for mitotic counting, which is a critical element of breast cancer diagnosis that is currently time consuming and subjective. In addition, Paige Breast Detect and Neoplasm enable pathologists to better prioritize their review of slides or entire cases by providing AI results throughout the diagnostic workflow to enhance efficiency. The Suite is built using the most advanced training techniques to be able to adapt to any lab without on-site tuning or calibration.
“At the heart of every Paige offering is the highest level of patient care,” said David Klimstra, M.D., Founder and Chief Medical Officer at Paige. “With the addition of Neoplasm and Mitosis detection to our Breast Suite, we assist pathologists in gaining greater efficiency and confidence in everyday tasks like mitotic counting. There are too few pathologists for the increasing demands of breast cancer diagnosis. Paige tackles the pathologist shortage by supporting breast cancer diagnosis and simultaneously providing greater confidence, reducing false negatives and increasing pathologists’ efficiency.”
The Paige Breast Suite is clinical-grade and built to the highest regulatory standards. Leveraging the same core technology that earned Paige FDA-approval for Paige Prostate Detect*, which was built on thousands of slides from hundreds of global institutions, the Paige Breast Suite delivers high performance across diverse datasets and is fit for use in real-world clinical settings.
* In the United States, Paige Prostate Detect (DEN200080) is approved for clinical use with Philips Ultrafast Scanner.
**In European Union and United Kingdom, Paige Breast Suite AI Applications are CE-IVD & UKCA marked for clinical use with Leica Aperio AT2 and GT450 Scanners. In United States and where research use is permitted, Paige Breast Suite applications use are limited to Research Use Only and not for use in diagnostic procedures.